Part D. Chapter 5: Food Sustainability and Safety
Usual Caffeine Consumption and Health
Systematic Review Question: Total Mortality
What is the relationship between usual caffeine consumption and total mortality?
Conclusion Statement: Strong and consistent evidence shows that consumption of coffee within the moderate range (3 to 5 cups/d or up to 400 mg/d caffeine) is not associated with increased risk of major chronic diseases, such as cardiovascular disease (CVD) and cancer and premature death in healthy adults.
DGAC Grade: Strong
Key Findings
- Coffee consumption was associated with reduced risk of total mortality (3-4% lower mortality with 1 cup/day), especially cardiovascular mortality
- Decaffeinated coffee consumption was associated with a lower risk of death (5 studies only)
- The limited number of studies on decaffeinated coffee indicates that protective association of coffee consumption may not be due to caffeine alone
Description of the Evidence
Two systematic reviews and/or meta-analyses (SR/MAs) of 20 and 23 prospective cohort studies (Je 2013 and Malerba 2013, respectively). Je et al examined total mortality and Malerba et al examined total, CVD, and cancer mortality.
Evidence suggests a significant inverse relationship between coffee consumption of 1-4 cups/day with total mortality, especially cardiovascular disease mortality. This evidence is based on three meta-analyses of more than 20 prospective cohort studies (Je, 2013; Malerba, 2013; Crippa, 2014). In general, results were similar for men and women. The risk reduction associated with each cup of coffee per day was between 3-4 percent. In addition, Je (2013) found a significant inverse association between coffee consumption and cardiovascular disease mortality. This association was stronger in women (16% lower risk) than in men (8% lower risk). However, no association was found for cancer mortality. Crippa et al. found that the lowest risk was observed for 4 cups/d for all-cause mortality (16%, 95% CI = 13-18) and 3 cups/d for CVD mortality (21%, 95% CI = 16-26),
Systematic Review Question: Cardiovascular Disease
What is the relationship between usual caffeine consumption and cardiovascular disease?
Conclusion Statement: Consistent observational evidence indicates that moderate coffee consumption is associated with reduced risk of type 2 diabetes and cardiovascular disease in healthy adults. In addition, consistent observational evidence indicates that regular consumption of coffee is associated with reduced risk of cancer of the liver and endometrium, and slightly inverse or null associations are observed for other cancer sites.
DGAC Grade: Moderate
Key Findings
CVD
- Non-linear association between coffee intake and risk of CVD
- Moderate coffee consumption was inversely associated with CVD risk
- Lowest risk at 3-5 cups/d
- Heavy consumption was not associated with higher CVD risk
Stroke
- Non-linear association between coffee intake and risk of stroke
- Moderate coffee consumption was inversely associated with stroke
- Lowest risk at 3-4 cups/d
- Higher intakes were not associated with higher stroke risk
CHD
- Moderate coffee consumption was associated with lower CHD risk
- Higher intakes were not associated with higher CHD risk
Heart Failure
- Moderate (1-5 cups/d) coffee consumption was inversely associated with risk of heart failure
- The largest inverse association observed for 4 cups/d
Blood Pressure & Hypertension
- No effect of coffee on long-term BP or risk of HTN
- For habitual coffee consumption, consumption of >3 cups/d was not associated with increased risk of HTN compared with <1 cup/d
- There was a slightly elevated risk of HTN for light to moderate consumption (1-3 cups/d)
- In hypertensive individuals, caffeine intake produces an acute increase in BP for ≥3 h, but there is no evidence of an association between long-term coffee consumption and increased BP
- Regular caffeine intake (median 410 mg/d) increases BP in short-term RCTs, although when ingested through coffee, BP effect of caffeine was smaller but significant
Atrial Fibrillation
- Caffeine was not associated with increased risk of atrial fibrillation
- Low-dose caffeine exposure (<350 mg) may have a protective effect
Blood Lipids
- Caffeinated, but not decaffeinated coffee, had significant effect on serum lipids. The effects were mostly found in unfiltered coffee.
- Coffee consumption increased TC, LDL-C, and TG
- Positive dose-response relation between coffee intake and TC, LDL-C, and TG
Description of the Evidence
Twelve SR/MAs examined CVD (Ding 2014, Caldiera 2013, Cai 2013, Kim 2012, Mostofsky 2012, Steffen 2012, Zhang 2011, Mesas 2011, Larrson 2011, Wu 2009, Soffi 2007, Noordjiz 2005). Some SR/MAs covered only RCTs (Cai 2013). Others included only prospective cohort studies (Larsson 2011, Zhang 2011, Kim2012, Mostofsky 2012, Wu 2009). Other SR/MAs covered RCTs and cohort studies (Steffen 2012); controlled trials (randomized and non-randomized) and cohort studies (Mesas 2011); prospective studies and case-control (Soffi 2007); prospective cohort studies, case-cohort, and nested case-control studies (Ding 2014); and RCT, prospective or retrospective cohorts and case-control studies (Caldiera 2013). The number of studies included in the SR/MAs ranged from 5-36.
A large and current body of evidence directly addressed the relationship between normal coffee consumption and risk of cardiovascular disease (CVD). The evidence included 12 systematic reviews with meta-analyses, all of which had high quality ratings (AMSTAR scores 8/11 – 11/11). CVD incidence and mortality, as well as coronary heart disease (CHD), stroke, heart failure, and hypertension were assessed by meta-analyses that consisted primarily of prospective cohort studies; intermediate outcomes such as blood pressure, blood lipids, and blood glucose were assessed by meta-analyses of randomized controlled trials.
CVD risk was assessed by a current meta-analysis of 36 prospective cohort studies on long-term coffee consumption (Ding, 2014). This analysis showed a non-linear association, such that the lowest risk of CVD was seen with moderate coffee consumption (3-5 cups/day), but higher intakes (>5 cups/day) were neither protective nor harmful. Overall, moderate consumption of caffeinated, but not decaffeinated, coffee was associated with a 12 percent lower risk of CVD.
Results from the assessment of CHD risk in three meta-analyses (Ding, 2014; Wu, 2009; Sofi, 2007) were inconsistent. Ding (2014) found 10 percent lower CHD risk with moderate coffee consumption (3-5 cups/day) in a meta-analysis of 30 prospective cohort studies, whereas Wu (2009) and Sofi (2007) in meta-analyses of 21 and 10 prospective cohort studies, respectively, found no association between coffee consumption and CHD risk. However, in sub-group analysis, Wu (2009) found that habitual moderate coffee consumption (1-4 cups/day) was associated with an 18 percent lower risk among women. Overall, the meta-analyses of Sofi (2007) and Wu (2009) were conducted with smaller bodies of evidence and Ding (2014) assessed several more recent studies. One reason for the inconsistent associations may be that coffee brewing methods have changed over time and the filter method has become more widely used, replacing unfiltered forms of coffee such as boiled coffee that were more widely consumed by participants in earlier studies.
Risk of stroke was assessed in two systematic reviews with meta-analyses of prospective cohort studies (Larsson, 2011; Kim, 2012) with consistent findings. Kim (2012) found that coffee intake of 4 or more cups/day had a protective effect on risk of stroke. Larsson (2011) documented a non-linear association such that coffee consumption ranging from 1 to 6 cups/day was associated with an 8 percent-13 percent lower risk of stroke, and higher intakes were not associated with decreased or increased risk. The inverse associations were limited to ischemic stroke and no association was seen with hemorrhagic stroke.
Regarding blood pressure, three meta-analyses evaluated the effect of coffee and caffeine on systolic and diastolic blood pressure using controlled trials (Steffen, 2012; Mesas, 2011; Noordzij, 2005). The most recent meta-analysis of 10 randomized controlled trials by Steffen et al. (2012) showed no effect of coffee on either systolic or diastolic blood pressure. Similarly, in another meta-analysis of 11 coffee trials and 5 caffeine trials, caffeine doses of <410 mg/day had no effect on systolic and diastolic blood pressure while doses of 410 or more mg/day resulted in a net increase (Noordzij, 2005). A third meta-analysis showed that among individuals with hypertension, 200-300 mg of caffeine (equivalent to ~2-3 cups filtered coffee) resulted in an acute increase of systolic and diastolic blood pressure (Mesas, 2011). Additionally, two meta-analyses quantified the effect of coffee on incidence of hypertension (Steffen, 2012; Zhang, 2011) and found no association between habitual coffee consumption and risk of hypertension. However, Zhang et al. (2011) documented a slightly elevated risk for light to moderate consumption (1-3 cups/day) of coffee compared to less than 1 cup/day. Regarding blood lipids, in a quantitative analysis of short-term randomized controlled trials, Cai et al. (2012) revealed that coffee consumption contributed significantly to an increase in total cholesterol, LDL-cholesterol, and triglycerides, and that unfiltered coffee had a greater effect than filtered coffee. Interestingly, caffeinated, but not decaffeinated (more likely to be filtered), coffee had this effect on serum lipids.
In a meta-analysis of observational study data, including prospective, retrospective, and case-control studies, higher amounts of coffee or caffeine had no association with risk of atrial fibrillation, but low doses of caffeine (<350 mg/day) appeared to have a protective effect (Caldeira, 2013). In contrast, coffee consumption of 1-5 cups/day was found to be inversely associated with risk of heart failure in a meta-analysis of 5 prospective studies (Mostofsky, 2012). A non-linear association was documented and the lowest risk was observed for 4 cups/day (Mostofsky, 2012).
Systematic Review Question: Type 2 Diabetes
What is the relationship between usual caffeine consumption and type 2 diabetes?
Conclusion Statement: Consistent observational evidence indicates that moderate coffee consumption is associated with reduced risk of type 2 diabetes and cardiovascular disease in healthy adults. In addition, consistent observational evidence indicates that regular consumption of coffee is associated with reduced risk of cancer of the liver and endometrium, and slightly inverse or null associations are observed for other cancer sites.
DGAC Grade: Moderate
Key Findings
- Coffee consumption was inversely associated with T2D risk in a dose-response manner
- Both caffeinated and decaffeinated coffee were associated with lower T2D risk
- Increased coffee consumption by 1 cup/d was associated with 7% lower T2D risk
- Similar associations were seen in men and women
- A smaller number of studies on decaffeinated coffee indicate that protective association of coffee consumption is unlikely to be due to caffeine alone
- In T2D individuals, ingestion of caffeine (~200-500 mg) significantly increased blood glucose, serum insulin, and lowered insulin sensitivity in those with T2D in short-term RCTs.
Description of the Evidence
Five SR/MAs examined T2D (Ding 2014, Jiang 2014, Whitehead 2013, Huxley 2009, Van Dam 2005). One SR/MA covered controlled trials (Whitehead 2013) and two others covered only prospective cohort studies (Jiang 2014, Huxley 2009). Other SR/MAs covered both prospective cohort and nested case-control studies (Ding 2014) or prospective cohort and cross-sectional studies (van Dam 2005). The number of studies included in the SR/MAs ranged from 9-31.
Coffee consumption has consistently been associated with a reduced risk of type 2 diabetes. In four meta-analyses of prospective cohort studies (Ding, 2014; Jiang, 2014; Huxley, 2009; van Dam, 2005) and cross-sectional studies (van Dam, 2005), coffee consumption was inversely associated with risk of type 2 diabetes in a dose-response manner. Risk for type 2 diabetes was 33 percent lower for those consuming 6 cups/day in the analysis by Ding et al. (2014) while the risk was 37 percent lower for those consuming 10 cups/day in the analysis by Jiang et al. (2014). Using a sub-set of the prospective cohorts in the Ding et al. (2014) and Jiang et al. (2014) meta-analyses, Huxley (2009) documented that each cup of coffee was associated with a 7 percent lower risk of type 2 diabetes. Similarly, van Dam (2005) noted that consumption of ≥6 or ≥7 cups/day was associated with a 35 percent lower risk of type 2 diabetes. Three meta-analyses (Ding, 2014; Jiang, 2014; Huxley, 2009) found protective associations for decaffeinated coffee. Moderate decaffeinated coffee consumption (3-4 cups/day) was associated with a 36 percent lower risk of type 2 diabetes (Huxley, 2009). Each cup of decaffeinated coffee was associated with a 6 percent lower risk (Ding, 2014) while every 2 cups were associated with a 11 percent lower risk (Jiang, 2014). Both reports also documented a dose-response association between caffeine and type 2 diabetes risk such that every 140 mg/day was associated with an 8 percent lower risk in the Ding et al (2014) meta-analysis while every 200 mg/day was associated with a 14 percent lower risk in the analysis by Jiang et al (2014). However, it remains unclear if this inverse association is independent of coffee consumption as Ding et al (2014) indicated that none of the studies included in the caffeine dose-response analysis adjusted for total coffee.
Only one systematic review of 9 randomized controlled trials examined the effects of caffeine on blood glucose and insulin concentrations among those with type 2 diabetes (Whitehead & White 2013). Ingestion of 200-500 mg of caffeine acutely increased blood glucose concentrations by 16-28 percent of the area under the curve and insulin secretions by 19-48 percent of the area under the curve when taken prior to a glucose load. At the same time, these trials also noted a decrease in insulin sensitivity by 14-37 percent. Although it is not clear if the acute effects of caffeine on blood glucose and insulin persist in the long term, evidence from prospective cohorts indicate that caffeine may have no adverse effect on the risk of type 2 diabetes.
Systematic Review Question: Cancer
What is the relationship between usual caffeine consumption and cancer?
Conclusion Statement: Consistent observational evidence indicates that moderate coffee consumption is associated with reduced risk of type 2 diabetes and cardiovascular disease in healthy adults. In addition, consistent observational evidence indicates that regular consumption of coffee is associated with reduced risk of cancer of the liver and endometrium, and slightly inverse or null associations are observed for other cancer sites.
DGAC Grade: Moderate
Key Findings
Total Cancer
- Total Cancer Coffee drinkers had a modestly lower total cancer incidence compared to nondrinkers or those with the lowest intakes
Lung Cancer
- Coffee consumption was associated with higher risk of lung cancer, but the association was mainly explained by smoking. An association was not founder among nonsmokers
Liver Cancer
- Significant inverse association between coffee consumption and liver cancer risk seen in both case-control and cohort studies (after adjustment for existing liver disease)
- Risk of hepatocelluar carcinoma was reduced by 40% for any coffee consumption versus no coffee consumption
Breast Cancer
- No association between caffeine, coffee, or decaffeinated coffee and breast cancer risk.
- An inverse association was seen in postmenopausal women and a strong inverse association seen in BRCA1 mutation carriers
- Borderline lower risk for highest versus lowest coffee consumption
- For all studies together, an increase of 2 cups of coffee per day was associated with a 2% marginally lower breast cancer risk
Prostate Cancer
- Regular coffee consumption associated with modestly lower risk of prostate cancer
- Significant inverse association documented for cohort studies. For case-control studies, a 2 cup increment was associated with a higher risk of prostate cancer
- Dose-response meta-analysis of coffee consumption showed inverse association with prostate cancer mortality, but not incidence
Ovarian Cancer
- No association between coffee consumption and ovarian cancer risk in high versus low or dose-response meta-analysis
Endometrial Cancer
- Increased coffee intake was associated with a reduced risk of endometrial cancer in both cohort and case-control studies
- A reduction of ~20% in endometrial cancer risk among coffee drinkers; >20% and >30% reduction in risk among low to mod and heavy drinkers, respectively
Bladder Cancer
- Data from case-control studies suggest that consumption of coffee is associated with an increased risk for bladder cancer, but no significant association was seen in prospective cohort studies
Pancreatic Cancer
- Meta-analysis of prospective cohort studies showed that coffee drinking was inversely associated with pancreatic cancer risk (in sub-group analyses, there was a reduced risk in men but not women)
- A positive association was found between coffee intake and pancreatic cancer in case-control studies that did not adjust for smoking. An inverse association was found in prospective cohort studies.
Upper Digestive & Respiratory Cancer
- Coffee drinking was inversely related to oral/pharyngeal cancer risk while there was no relation with laryngeal cancer, ESCC, and EAC
Gastro-esophageal Cancer
- Coffee consumption was inversely, but non-significantly, associated with risk of esophageal cancer
- No association between coffee consumption and gastric cancer risk in cohort or case-control studies
Colorectal Cancer
- Case-control studies suggest coffee consumption decreases risk of colorectal and colon cancer, especially in women; the association was inverse, but marginally non-significant, for cohort studies for colorectal and colon cancer
- Prospective cohort studies showed no association between coffee consumption on colorectal cancer risk (a suggestive inverse association was slightly stronger in studies that adjusted for smoking and alcohol)
Description of the Evidence
A large number of SR/MAs addressed cancer, including total cancer (Yu 2011), lung cancer (Tang 2010), liver cancer (Sang 2013, Bravi 2013), breast cancer (Jiang 2013, Li 2013, Tang 2009), prostate cancer (Cao 2014, Zhong 2013, Discacciati 2013, Park 2010), ovarian cancer (Braem 2012), endometrial cancer (Je 2012, Bravi 2009), bladder cancer (Zhou 2012), pancreatic cancer (Turati 2011, Dong 2011), upper digestive and respiratory tract cancer (Turati 2011), esophageal cancer (Zheng 2013), gastric cancer (Botelho 2006), and colorectal cancer (Li 2012, Galeone 2010, Je 2009). The majority of the studies included cohort and cross-sectional studies, although some covered only prospective cohort studies or case-control studies. The number of studies included in the SR/MAs ranged from 3-54.
Several systematic reviews and meta-analyses examined the association between coffee consumption and risk of cancer. Types of cancer examined by the Committee included total cancer, cancers of the lung, liver, breast, prostate, ovaries, endometrium, bladder, pancreas, upper digestive and respiratory tract, esophagus, stomach, colon, and rectum.
In a quantitative summary of 40 prospective cohort studies with an average follow-up of 14.3 years, Yu (2011) found a 13 percent lower risk of total cancer among coffee drinkers compared to non-drinkers or those with lowest intakes. Risk estimates were similar for men and women. In sub-group analyses, the authors noted that coffee drinking was associated with a reduced risk of bladder, breast, buccal and pharyngeal, colorectal, endometrial, esophageal, hepatocellular, leukemic, pancreatic, and prostate cancers.
Tang et al (2010) evaluated 5 prospective cohorts and 8 case-control studies and found that overall those with the highest levels of coffee consumption had a 27 percent higher risk for lung cancer compared to never drinkers or those with least consumption. An increase in coffee consumption of 2 cups/day was associated with a 14 percent higher risk of developing lung cancer. However, because smoking is an important confounder, when analyses were stratified by smoking status, coffee consumption was marginally protective in non-smokers and was not associated with lung cancer among smokers. When estimates from 2 studies that examined decaffeinated coffee were summarized, there was a protective association with lung cancer. No association was seen with lung cancer when only case-control studies were considered.
Results from two meta-analyses indicate the coffee consumption is associated with a 50 percent lower risk of liver cancer (Sang, 2013) and a 40 percent lower risk of hepatocellular carcinoma (Bravi, 2013) when considering both cohort and case-control studies. Associations were significant in men but not in women (Sang, 2013).
Three meta-analyses of observational studies found no association between coffee consumption (Jiang, 2013; Li, 2013; Tang, 2013), caffeine consumption (Jiang, 2013), or decaffeinated coffee consumption (Jiang, 2013) and risk of breast cancer. In all 3 reports, each 2 cup/day of coffee was marginally associated with a 2 percent lower risk of breast cancer. However, in sub-group analyses, coffee consumption was protective against breast cancer risk in postmenopausal women (Jiang, 2013), BRCA1 mutation carriers (Jiang, 2013), and women with estrogen receptor negative status (Li, 2013).
The association between coffee consumption and risk of prostate cancer was mixed. Cao (2014) and Zhong (2013) found that regular or high coffee consumption, compared to non- or lowest levels of consumption, was associated with a 12 percent-17 percent lower risk of prostate cancer in prospective cohort studies. Further, each 2 cups of coffee per day was associated with a 7% lower risk of prostate cancer. However, no associations were seen with case-control data alone or when these studies were examined together with prospective cohort studies. Using a combination of both prospective cohort and case-control data, Discacciati (2013) found that each 3 cups/day of coffee was associated with a 3% lower risk of localized prostate cancer and an 11% lower risk of mortality from prostate cancer. On the other hand, after summarizing data from 12 prospective cohort and case-control studies, Park (2010) found a 16% higher risk of prostate cancer. However, in sub-group analyses by study design, the higher risk was observed in case-control but not in cohort studies.
Consumption of coffee was not associated with risk of ovarian cancer in a meta-analysis of 7 prospective cohort studies with over 640,000 participants (Braem, 2012).
Two meta-analyses confirmed an inverse association between coffee consumption and risk of endometrial cancer (Je, 2012; Bravi, 2009). In the most recent and updated meta-analysis of prospective cohort and case-control studies, compared to those in the lowest category of coffee consumption, those with the highest intakes of coffee had a 29% lower risk of endometrial cancer (Je, 2012). Each cup of coffee per day was associated with an 8% lower risk of endometrial cancer. Similar results were found in the meta-analysis by Bravi (2009) that included a sub-set of the studies in Je (2012) and documented a 20% lower risk of endometrial cancer overall, and a 7% decrease for each cup of coffee per day. However, the association was significant only in case-control studies but not in cohort studies, most likely due to lower statistical power.
A recent meta-analysis of 23 case-control studies by Zhou (2012) found coffee was a risk factor for bladder cancer. There was a smoking-adjusted increased risk of bladder cancer for those in the highest (45%), second highest, (21%), and third highest (8%) groups of coffee consumption, compared to those in the lowest group. No association was, however, seen in cohort studies.
Two meta-analyses of coffee consumption and pancreatic cancer risk provided mixed results (Turati, 2011; Dong, 2011). Using both prospective cohort and case-control studies, Turati (2011) found that coffee consumption was not associated with risk of pancreatic cancer. However, an increased risk was seen in case-control studies that did not adjust for smoking. Using a sub-set of prospective cohorts included in the Turati (2011) meta-analysis, Dong (2011) found that coffee drinking was inversely associated with pancreatic cancer risk but did not separate studies based on their adjustment for smoking status. Sub-group analyses revealed a protective association in men, but not in women.
Turati (2011) quantified the association between coffee consumption and various upper digestive and respiratory tract cancers using data from observational studies. Coffee consumption was associated with a 36% lower risk of oral and pharyngeal cancer but not with risk of laryngeal cancer, esophageal squamous cell carcinoma, or esophageal adenocarcinoma. In a meta-analysis of prospective cohort and case-control studies, Zheng (2013) noted that coffee was inversely, but non-significantly, associated with risk of esophageal cancer. Regarding gastric cancer, no association between coffee consumption and risk was seen in a meta-analysis of observational studies by Botelho (2006).
Three meta-analyses on the association between coffee consumption and colorectal cancer risk (Li, 2012; Galeone, 2012; Je, 2009) have yielded mixed findings. Results from case-control studies suggested coffee consumption was associated with lower risk of colorectal (15% lower) and colon cancer (21% lower), especially in women. However, this inverse association was non-significant for cohort studies. Using all but one of the case-control studies, Galeone (2012) arrived at similar conclusions as the Li (2012) analysis although associations were in general stronger. Galeone (2012) also provided suggestive evidence for a dose-response relationship between coffee and colorectal cancer such that each cup of coffee was associated with a 6% lower risk of colorectal cancer, 5% lower risk of colon cancer, and 3% lower risk of rectal cancer. Using several prospective cohort studies as in the Li (2012) meta-analysis, Je (2009) found no significant association of coffee consumption with risk of colorectal cancer. Interestingly, no differences were seen by sex but the suggestive inverse associations were slightly stronger in studies that adjusted for smoking and alcohol.
Systematic Review Question: Cognitive Function
What is the relationship between usual caffeine consumption and cognitive function?
Conclusion Statement: Limited evidence indicates that caffeine consumption is associated with a modestly lower risk of cognitive decline or impairment and lower risk of Alzheimer’s disease.
DGAC Grade: Limited
Key Findings:
- There was a trend toward a protective effect of caffeine from different sources and cognitive impairment/dementia.
Description of the Evidence
Two systematic reviews (Arab, 2013; Santos, 2010) and one meta-analysis (Santos, 2010) examined the effects of caffeine from various sources, including coffee, tea, chocolate, on cognitive outcomes. Arab (2013) systematically reviewed six longitudinal cohort studies evaluating the effect of caffeine or caffeine-rich beverages on cognitive decline. Most studies in this review used the Mini Mental State Examination Score as a global measure of cognitive decline. The review concluded that estimates of cognitive decline were lower among consumers, although there was no clear dose-response relationship. Studies also showed stronger effects among women than men. In a meta-analysis of nine cohort and two case-control studies, caffeine intake from various sources was associated with a 16% lower risk of various measures of cognitive impairment/decline. Specifically, data from four studies indicate that caffeine is associated with a 38% lower risk of Alzheimer’s disease.
Systematic Review Question: Parkinson’s Disease
What is the relationship between usual caffeine consumption and Parkinson’s disease?
Conclusion Statement: Consistent evidence indicates an inverse association between caffeine intake and risk of Parkinson’s disease.
DGAC Grade: Moderate
Key Findings
- There was a non-linear inverse association between coffee and Parkinson’s disease risk with maximum protection at ~3 cups/d (adjusted for smoking)
- For caffeine consumption, a linear inverse association was found (adjusted for smoking); every 300 mg/day was associated with a 24% lower risk of Parkinson’s disease.
Description of the Evidence
Evidence from two systematic reviews (Ishihara, 2005; Costa, 2010) and one quantitative meta-analysis (Qi, 2013) confirmed an inverse association between coffee, caffeine, and risk of Parkinson’s disease. Qi (2013) evaluated six case-control studies and seven prospective articles and documented a non-linear relationship between coffee and risk of Parkinson’s disease, overall. The lowest risk was observed at ~3 cups/day (smoking-adjusted risk reduction was 28%). For caffeine, a linear dose-response was found and every 200 mg/day increment in caffeine intake was associated with a 17% lower risk of Parkinson’s disease. Using a combination of cohort, case-control, and cross-sectional data, Costa (2010) summarized that the risk of Parkinson’s disease was 25% lower among those consuming the highest versus lowest amounts of caffeine. Like Qi (2013), Costa documented a linear dose-response with caffeine intake such that every 300 mg/day was associated with a 24% lower risk of Parkinson’s disease. In both reports, associations were weaker among women than in men.
Systematic Review Question: Pregnancy outcomes
What is the relationship between usual caffeine consumption and pregnancy outcomes?
Conclusion
Consistent evidence from observational studies indicates that caffeine intake in pregnant women is not associated with risk of preterm delivery. Higher caffeine intake (especially >=300 mg/day ) is associated with a small increased risk of miscarriage, stillbirth, low birth weight, and small for gestational age (SGA) births. However, these data should be interpreted cautiously due to potential recall bias in the case-control studies and confounding by smoking and pregnancy signal symptoms.
DGAC Grade Moderate
Key Findings
- No important association between caffeine intake during pregnancy and risk of pre-term birth were observed in either cohort or case-control studies.
- Consumption of caffeine from various sources was associated with a significantly increased risk of spontaneous abortion and low birth weight. Control for confounders such as maternal age, smoking, and ethanol use was not possible.
Description of the Evidence
Two SR/MAs assessed observational studies on the association of caffeine intake with adverse pregnancy outcomes (Greenwood 2014, Maslova 2010). The pregnancy outcomes included miscarriage, pre-term birth, stillbirth, small for gestational age (SGA), and low birth-weight. The most recent SR/MA by Greenwood et al quantified the association between caffeine intake and adverse pregnancy outcomes from 60 publications from 53 separate cohort (26) and case-control (27) studies. The evidence covered a variety of countries with caffeine intake categories that ranged from non-consumers to those consuming >1,000mg/day. They found that an increment of 100 mg caffeine was associated with a 14% increased risk of miscarriage, 19% increased risk of stillbirth, 10% increased risk of SGA, and 7% increased risk of low birth weight. There was no significant increase in risk of preterm delivery. The magnitude of these associations was relatively small within the range of caffeine intakes of the majority women in the study populations, and the associations became more pronounced at higher range (>=300 mg/day). The authors also note the substantial heterogeneity observed in the meta-analyses shows that interpretation of the results should be cautious. In addition, the results from prospective cohort studies and case-control studies were mixed together. Since coffee consumption is positively correlated with smoking, residual confounding by smoking may have biased the results toward a positive direction.
The other SR/MAs did not cover all of the above pregnancy outcomes, but for those adverse outcomes covered, the results were in agreement with Greenwood et al. Maslova (2010) reviewed 22 studies (15 cohort and 7 case-control studies) and found no significant association between caffeine intake and risk of pre-term birth in either case-control or cohort studies. For all of the observational studies assessed across the three SR/MAs, most studies did not adequately adjust for the pregnancy signal phenomenon, i.e. that nausea, vomiting, and other adverse symptoms are associated with a healthy pregnancy that results in a live birth, whereas pregnancy signal symptoms occur less frequently when the result is miscarriage. Coffee consumption decreases with increasing pregnancy signal symptoms, typically during the early weeks of pregnancy, and this confounds the association (Peck et al 2010). Greenwood et al state that this potential bias is the most prominent argument against a causal role for caffeine in adverse pregnancy outcomes. Only one randomized controlled trial of caffeine/coffee reduction during pregnancy has been conducted to date (Bech 2007). The study found that a reduction of 200 mg of caffeine intake per day did not significantly influence birth weight or length of gestation. The trial did not examine other outcomes.
Research Recommendations
- Evaluate the effects of coffee on health outcomes in vulnerable populations, such as women who are pregnant (premature birth, low birth weight, spontaneous abortion).
- Examine the effects of coffee on sleep patterns, quality of life, and dependency and addiction.
- Evaluate the prospective association between coffee/caffeine consumption and cancer at different sites.
- Examine prospectively the effects of coffee/caffeine on cognitive decline, neurodegenerative diseases, and depression.
- Understand the mechanisms underlying the protective effects of coffee on diabetes and CVD.
- Understand the association between coffee and health outcomes in individuals with existing CVD, diabetes, cancer, neurodegenerative diseases, or depressive symptoms.
References
- Crippa A, Discacciati A, Larsson SC, Wolk A, Orsini N. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol. 2014;180(8):763-75. PMID: 25156996. http://www.ncbi.nlm.nih.gov/pubmed/25156996.
- Je Y, Giovannucci E. Coffee consumption and total mortality: a meta-analysis of twenty prospective cohort studies. Br J Nutr. 2014;111(7):1162-73. PMID: 24279995. http://www.ncbi.nlm.nih.gov/pubmed/24279995.
- Malerba S, Turati F, Galeone C, Pelucchi C, Verga F, La Vecchia C, et al. A meta-analysis of prospective studies of coffee consumption and mortality for all causes, cancers and cardiovascular diseases. Eur J Epidemiol. 2013;28(7):527-39. PMID: 23934579. http://www.ncbi.nlm.nih.gov/pubmed/23934579.
- Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129(6):643-59. PMID: 24201300. http://www.ncbi.nlm.nih.gov/pubmed/24201300.
- Sofi F, Conti AA, Gori AM, Eliana Luisi ML, Casini A, Abbate R, et al. Coffee consumption and risk of coronary heart disease: a meta-analysis. Nutr Metab Cardiovasc Dis. 2007;17(3):209-23. PMID: 17156982. http://www.ncbi.nlm.nih.gov/pubmed/17156982.
- Wu JN, Ho SC, Zhou C, Ling WH, Chen WQ, Wang CL, et al. Coffee consumption and risk of coronary heart diseases: a meta-analysis of 21 prospective cohort studies. Int J Cardiol. 2009;137(3):216-25. PMID: 18707777. http://www.ncbi.nlm.nih.gov/pubmed/18707777.
- Kim B, Nam Y, Kim J, Choi H, Won C. Coffee Consumption and Stroke Risk: A Meta-analysis of Epidemiologic Studies. Korean J Fam Med. 2012;33(6):356-65. PMID: 23267421. http://www.ncbi.nlm.nih.gov/pubmed/23267421.
- Larsson SC, Orsini N. Coffee consumption and risk of stroke: a dose-response meta-analysis of prospective studies. Am J Epidemiol. 2011;174(9):993-1001. PMID: 21920945. http://www.ncbi.nlm.nih.gov/pubmed/21920945.
- Mesas AE, Leon-Munoz LM, Rodriguez-Artalejo F, Lopez-Garcia E. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: a systematic review and meta-analysis. Am J Clin Nutr. 2011;94(4):1113-26. PMID: 21880846. http://www.ncbi.nlm.nih.gov/pubmed/21880846.
- Noordzij M, Uiterwaal CS, Arends LR, Kok FJ, Grobbee DE, Geleijnse JM. Blood pressure response to chronic intake of coffee and caffeine: a meta-analysis of randomized controlled trials. J Hypertens. 2005;23(5):921-8. PMID: 15834273. http://www.ncbi.nlm.nih.gov/pubmed/15834273.
- Steffen M, Kuhle C, Hensrud D, Erwin PJ, Murad MH. The effect of coffee consumption on blood pressure and the development of hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30(12):2245-54. PMID: 23032138. http://www.ncbi.nlm.nih.gov/pubmed/23032138.
- Zhang Z, Hu G, Caballero B, Appel L, Chen L. Habitual coffee consumption and risk of hypertension: a systematic review and meta-analysis of prospective observational studies. Am J Clin Nutr. 2011;93(6):1212-9. PMID: 21450934. http://www.ncbi.nlm.nih.gov/pubmed/21450934.
- Cai L, Ma D, Zhang Y, Liu Z, Wang P. The effect of coffee consumption on serum lipids: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2012;66(8):872-7. PMID: 22713771. http://www.ncbi.nlm.nih.gov/pubmed/22713771.
- Jee SH, He J, Appel LJ, Whelton PK, Suh I, Klag MJ. Coffee consumption and serum lipids: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol. 2001;153(4):353-62. PMID: 11207153. http://www.ncbi.nlm.nih.gov/pubmed/11207153.
- Caldeira D, Martins C, Alves LB, Pereira H, Ferreira JJ, Costa J. Caffeine does not increase the risk of atrial fibrillation: a systematic review and meta-analysis of observational studies. Heart. 2013;99(19):1383-9. PMID: 24009307. http://www.ncbi.nlm.nih.gov/pubmed/24009307.
- Mostofsky E, Rice MS, Levitan EB, Mittleman MA. Habitual coffee consumption and risk of heart failure: a dose-response meta-analysis. Circ Heart Fail. 2012;5(4):401-5. PMID: 22740040. http://www.ncbi.nlm.nih.gov/pubmed/22740040.
- Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care. 2014;37(2):569-86. PMID: 24459154. http://www.ncbi.nlm.nih.gov/pubmed/24459154.
- Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169(22):2053-63. PMID: 20008687. http://www.ncbi.nlm.nih.gov/pubmed/20008687.
- Jiang X, Zhang D, Jiang W. Coffee and caffeine intake and incidence of type 2 diabetes mellitus: a meta-analysis of prospective studies. Eur J Nutr. 2014;53(1):25-38. PMID: 24150256. http://www.ncbi.nlm.nih.gov/pubmed/24150256.
- van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. Jama. 2005;294(1):97-104. PMID: 15998896. http://www.ncbi.nlm.nih.gov/pubmed/15998896.
- Whitehead N, White H. Systematic review of randomised controlled trials of the effects of caffeine or caffeinated drinks on blood glucose concentrations and insulin sensitivity in people with diabetes mellitus. J Hum Nutr Diet. 2013;26(2):111-25. PMID: 23331476. http://www.ncbi.nlm.nih.gov/pubmed/23331476.
- Yu X, Bao Z, Zou J, Dong J. Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer. 2011;11:96. PMID: 21406107. http://www.ncbi.nlm.nih.gov/pubmed/21406107.
- Tang N, Wu Y, Ma J, Wang B, Yu R. Coffee consumption and risk of lung cancer: a meta-analysis. Lung Cancer. 2010;67(1):17-22. PMID: 19362749. http://www.ncbi.nlm.nih.gov/pubmed/19362749.
- Sang LX, Chang B, Li XH, Jiang M. Consumption of coffee associated with reduced risk of liver cancer: a meta-analysis. BMC Gastroenterol. 2013;13:34. PMID: 23433483. http://www.ncbi.nlm.nih.gov/pubmed/23433483.
- Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol. 2013;11(11):1413-21.e1. PMID: 23660416. http://www.ncbi.nlm.nih.gov/pubmed/23660416.
- Jiang W, Wu Y, Jiang X. Coffee and caffeine intake and breast cancer risk: an updated dose-response meta-analysis of 37 published studies. Gynecol Oncol. 2013;129(3):620-9. PMID: 23535278. http://www.ncbi.nlm.nih.gov/pubmed/23535278.
- Li XJ, Ren ZJ, Qin JW, Zhao JH, Tang JH, Ji MH, et al. Coffee consumption and risk of breast cancer: an up-to-date meta-analysis. PLoS One. 2013;8(1):e52681. PMID: 23308117. http://www.ncbi.nlm.nih.gov/pubmed/23308117.
- Tang N, Zhou B, Wang B, Yu R. Coffee consumption and risk of breast cancer: a metaanalysis. Am J Obstet Gynecol. 2009;200(3):290.e1-9. PMID: 19114275. http://www.ncbi.nlm.nih.gov/pubmed/19114275.
- Cao S, Liu L, Yin X, Wang Y, Liu J, Lu Z. Coffee consumption and risk of prostate cancer: a meta-analysis of prospective cohort studies. Carcinogenesis. 2014;35(2):256-61. PMID: 24343360. http://www.ncbi.nlm.nih.gov/pubmed/24343360.
- Zhong S, Chen W, Yu X, Chen Z, Hu Q, Zhao J. Coffee consumption and risk of prostate cancer: an up-to-date meta-analysis. Eur J Clin Nutr. 2014;68(3):330-7. PMID: 24300907. http://www.ncbi.nlm.nih.gov/pubmed/24300907.
- Discacciati A, Orsini N, Wolk A. Coffee consumption and risk of nonaggressive, aggressive and fatal prostate cancer--a dose-response meta-analysis. Ann Oncol. 2014;25(3):584-91. PMID: 24276028. http://www.ncbi.nlm.nih.gov/pubmed/24276028.
- Park CH, Myung SK, Kim TY, Seo HG, Jeon YJ, Kim Y. Coffee consumption and risk of prostate cancer: a meta-analysis of epidemiological studies. BJU Int. 2010;106(6):762-9. PMID: 20590551. http://www.ncbi.nlm.nih.gov/pubmed/20590551.
- Braem MG, Onland-Moret NC, Schouten LJ, Tjonneland A, Hansen L, Dahm CC, et al. Coffee and tea consumption and the risk of ovarian cancer: a prospective cohort study and updated meta-analysis. Am J Clin Nutr. 2012;95(5):1172-81. PMID: 22440851. http://www.ncbi.nlm.nih.gov/pubmed/22440851.
- Bravi F, Scotti L, Bosetti C, Gallus S, Negri E, La Vecchia C, et al. Coffee drinking and endometrial cancer risk: a metaanalysis of observational studies. Am J Obstet Gynecol. 2009;200(2):130-5. PMID: 19110217. http://www.ncbi.nlm.nih.gov/pubmed/19110217.
- Je Y, Giovannucci E. Coffee consumption and risk of endometrial cancer: findings from a large up-to-date meta-analysis. Int J Cancer. 2012;131(7):1700-10. PMID: 22190017. http://www.ncbi.nlm.nih.gov/pubmed/22190017.
- Zhou Y, Tian C, Jia C. A dose-response meta-analysis of coffee consumption and bladder cancer. Prev Med. 2012;55(1):14-22. PMID: 22564775. http://www.ncbi.nlm.nih.gov/pubmed/22564775.
- Turati F, Galeone C, Edefonti V, Ferraroni M, Lagiou P, La Vecchia C, et al. A meta-analysis of coffee consumption and pancreatic cancer. Ann Oncol. 2012;23(2):311-8. PMID: 21746805. http://www.ncbi.nlm.nih.gov/pubmed/21746805.
- Dong J, Zou J, Yu XF. Coffee drinking and pancreatic cancer risk: a meta-analysis of cohort studies. World J Gastroenterol. 2011;17(9):1204-10. PMID: 21448427. http://www.ncbi.nlm.nih.gov/pubmed/21448427.
- Turati F, Galeone C, La Vecchia C, Garavello W, Tavani A. Coffee and cancers of the upper digestive and respiratory tracts: meta-analyses of observational studies. Ann Oncol. 2011;22(3):536-44. PMID: 20943597. http://www.ncbi.nlm.nih.gov/pubmed/20943597.
- Zheng JS, Yang J, Fu YQ, Huang T, Huang YJ, Li D. Effects of green tea, black tea, and coffee consumption on the risk of esophageal cancer: a systematic review and meta-analysis of observational studies. Nutr Cancer. 2013;65(1):1-16. PMID: 23368908. http://www.ncbi.nlm.nih.gov/pubmed/23368908.
- Botelho F, Lunet N, Barros H. Coffee and gastric cancer: systematic review and meta-analysis. Cad Saude Publica. 2006;22(5):889-900. PMID: 16680342. http://www.ncbi.nlm.nih.gov/pubmed/16680342.
- Galeone C, Turati F, La Vecchia C, Tavani A. Coffee consumption and risk of colorectal cancer: a meta-analysis of case-control studies. Cancer Causes Control. 2010;21(11):1949-59. PMID: 20680435. http://www.ncbi.nlm.nih.gov/pubmed/20680435.
- Je Y, Liu W, Giovannucci E. Coffee consumption and risk of colorectal cancer: a systematic review and meta-analysis of prospective cohort studies. Int J Cancer. 2009;124(7):1662-8. PMID: 19115212. http://www.ncbi.nlm.nih.gov/pubmed/19115212.
- Li G, Ma D, Zhang Y, Zheng W, Wang P. Coffee consumption and risk of colorectal cancer: a meta-analysis of observational studies. Public Health Nutr. 2013;16(2):346-57. PMID: 22694939. http://www.ncbi.nlm.nih.gov/pubmed/22694939.
- Costa J, Lunet N, Santos C, Santos J, Vaz-Carneiro A. Caffeine exposure and the risk of Parkinson's disease: a systematic review and meta-analysis of observational studies. J Alzheimers Dis. 2010;20 Suppl 1:S221-38. PMID: 20182023. http://www.ncbi.nlm.nih.gov/pubmed/20182023.
- Ishihara L, Brayne C. A systematic review of nutritional risk factors of Parkinson's disease. Nutr Res Rev. 2005;18(2):259-82. PMID: 19079910. http://www.ncbi.nlm.nih.gov/pubmed/19079910.
- Qi H, Li S. Dose-response meta-analysis on coffee, tea and caffeine consumption with risk of Parkinson's disease. Geriatr Gerontol Int. 2014;14(2):430-9. PMID: 23879665. http://www.ncbi.nlm.nih.gov/pubmed/23879665.
- Arab L, Khan F, Lam H. Epidemiologic evidence of a relationship between tea, coffee, or caffeine consumption and cognitive decline. Adv Nutr. 2013;4(1):115-22. PMID: 23319129. http://www.ncbi.nlm.nih.gov/pubmed/23319129.
- Santos C, Costa J, Santos J, Vaz-Carneiro A, Lunet N. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20 Suppl 1:S187-204. PMID: 20182026. http://www.ncbi.nlm.nih.gov/pubmed/20182026.
- Greenwood DC, Thatcher NJ, Ye J, Garrard L, Keogh G, King LG, et al. Caffeine intake during pregnancy and adverse birth outcomes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. 2014;29(10):725-34. PMID: 25179792. http://www.ncbi.nlm.nih.gov/pubmed/25179792.
- Maslova E, Bhattacharya S, Lin SW, Michels KB. Caffeine consumption during pregnancy and risk of preterm birth: a meta-analysis. Am J Clin Nutr. 2010;92(5):1120-32. PMID: 20844077. http://www.ncbi.nlm.nih.gov/pubmed/20844077
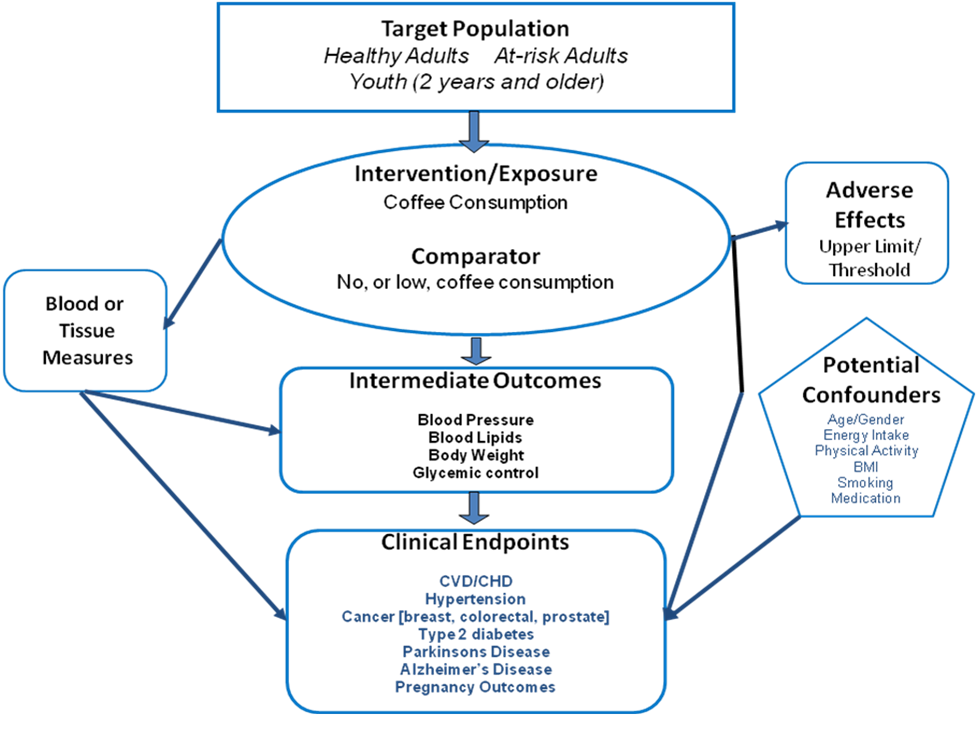
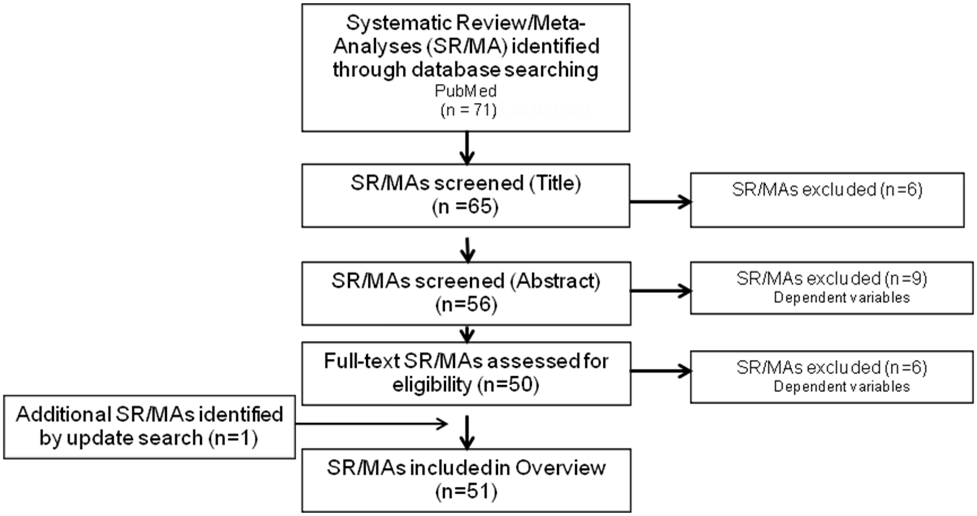
Analytical Framework
Inclusion Criteria
PubMed was searched for original research articles published in English in peer-reviewed journals. Studies published since January 2000 with subjects who were healthy or at elevated chronic disease risk from countries with high or very high human development were considered. Study design was limited to systematic reviews or systematic reviews with meta-analyses. All other study designs were excluded. Studies were required to specify level of caffeine and included caffeine from any source. Both short- and long-term health outcomes were included. Studies that examined low-calorie diets and other treatment diets were excluded. Finally, studies were required to include a description of the dietary pattern along with sustainability or food security outcomes.